Our vision is to conquer Alport syndrome, an important part of which is finding new treatments and a cure to prevent kidney failure and hearing loss in all patients with Alport syndrome.
Through our Research Program, funding is provided for basic science or clinical research on Alport syndrome. These funds may be used as seed funding for the testing of initial hypotheses and the collection of preliminary data leading to expanded funding by the National Institutes of Health (NIH) or other major funding institutions around the world. Information about ASF’s Research Funding Policies is available at this link.
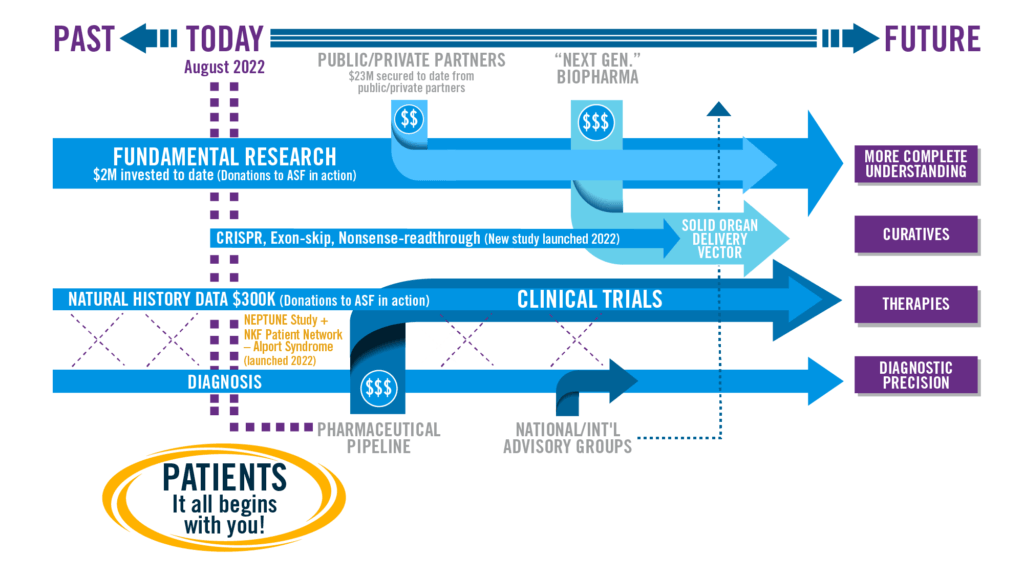
Continuing its strategic efforts from 2021 to target limited research funds to specific understudied aspects of Alport syndrome, ASF has selected not to offer a research competition in 2022. ASF is investing in the development of new and expanded research tools that have benefit for the entire community of Alport patients. Examples include the “NKF Patient Network – Alport Syndrome” (a patient registry) and a new natural history study in the form of an Ancillary Study in Alport syndrome in partnership with NEPTUNE at the University of Michigan. Note that ASF’s policy is to provide support for direct research costs only. Indirect research costs will not be supported through ASF’s Research Program.
2023 RESEARCH UPDATE (October 7th)
During our Alport Connect 2023 in-person meeting in San Diego (October 7-8), we recorded the Keynote Address on Hopeful Advances in Research, featuring remarks from ASF’s Research Director and members of our Scientific Advisory Research Network.
We are pleased to share this closed-captioned address. You can watch the entire recording, or use the chapters feature, located just below the video progress bar, to jump to speakers/topics of particular interest. Click the preview image above to view.
2022 RESEARCH OVERVIEW
ASF Volunteer Research Program Chair and Board of Directors member, B. André Weinstock, PhD, MSAS, provides an overview of the current Alport syndrome Research Roadmap in this closed-captioned video. A .PDF file containing all the Research Resources listed at the conclusion of the video can be found at this link.
2021 FUNDING AWARD

Dr. Felipe Santos, MD
April 22, 2021: Inspired by two Alport patients who reached out to us in 2020, ASF is making a new and historic investment of $26,000 to better understand the cause of Alport syndrome-related hearing loss. This is an important first step in understanding if and/or how Alport hearing loss may potentially be treated or cured.
The project is being led by Dr. Felipe Santos, a physician, surgeon, and Interim Chief of Otology and Neurotology at Massachusetts Eye and Ear. Dr. Santos is also an Assistant Professor of Otolaryngology – Head and Neck Surgery, at Harvard Medical School.
2021 FUNDING AWARD

Dr. Jeffrey Miner, PhD, FASN
UPDATE: In November 2021, Dr. Jeffrey Miner was awarded a $2.25 million grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to study Alport syndrome
March 18, 2021: Through the generosity of patients, families, and friends that donate to Alport Syndrome Foundation, ASF is honored to announce a 2021 funding award of $132,000 to Washington University’s Division of Nephrology in the Department of Medicine. These funds were awarded to Dr. Jeffrey Miner’s laboratory to perform proof of concept gene therapy studies in Alport mice. The studies will determine the ages and stages of kidney disease at which promising gene therapy approaches would be effective in Alport patients.
PREVIOUSLY FUNDED RESEARCH
Note: The 2019 and 2020 research projects were funded by Alport Syndrome Foundation.
AWARDED 2020
Sex Specific Genotype Penetrance for Predictive Diagnosis in Alport Syndrome
Drs. Moumita Barua and Andrew Paterson (University of Toronto)
Collaborators Dr. Moumita Barua and Dr.Andrew Paterson were collectively awarded $125,000 over two years for their project which takes research a step further in helping answer patients’ critical questions about disease progression patterns related to their specific sex and genetic mutation.
In their proposal, Drs. Barua and Paterson note Alport syndrome studies in the past have been biased toward individuals with more classic and severe manifestations. They hypothesize the prevalence of Alport syndrome is higher than previous estimates as a result of this inherent bias. Defining disease prevalence and genetic factors that influence its clinical spectrum will be achieved by studying participants in the UK Biobank, and patients with severe disease diagnosed in Canadian nephrology clinics. They will also focus on examining genetic factors that lead to clinical variability amongst females with X-linked Alport syndrome. Dr. Moumita Barua notes, “By the project’s completion, our work will define Alport syndrome prevalence while also investigating novel genetic modifiers for disease progression. In doing so, our findings will improve the ability to make diagnosis to facilitate early intervention and aid in the selection of high-risk patients for clinical trials.” Dr. Andrew Paterson says, “Large population-based studies along with detailed genetic data now allow us to examine how specific genetic variants in the genes related to Alport syndrome impact measures of kidney function in the general population.”
View the full 2020 Research Award Funding Press release here.
March 2022 UPDATE: Read the Final Report Summary for this Research Award (prepared by Drs. Barua and Paterson).
AWARDED 2019
Pharmaceutical Targeting of Alport Syndrome Modifier Genes
Dr. Ron Korstanje, Jackson Laboratory (Maine)
March 2022 UPDATE: Dr. Ron Korstanje, who leads a research team at The Jackson Laboratory, was awarded a $1.49M grant from the National Institute of Diabetes and Digestive and Kidney Disease to study Alport syndrome genetics.
Dr. Korstanje was awarded $125,00 over two years for his project which targets specific modifier genes that might reduce or delay the symptoms of Alport syndrome. There is large variation in the age of onset and severity of Alport syndrome in humans, even among patients with similar mutations. It is widely accepted that modifier genes contribute to this variation. Because modifier genes have been shown to be effective therapeutic targets in other diseases, they have potential to serve similarly in Alport syndrome. Using new mice models, Dr. Korstanje will determine if therapeutic intervention using a drug shown to reduce chronic kidney disease in other animal models could prolong kidney function.
Tubulointerstitial Profiling for Alport Syndrome Target Identification
Dr. Benjamin Humphreys, Washington University (Missouri)
Dr. Humphreys was awarded $125,000 over two years for his tubulointerstial profiling project. Dr. Humphreys has successfully mapped the complex protein “communication” pathways between the cells of the glomeruli and the cells of the neighboring tubules of the kidneys. His premise is that cell-specific profiling of the cell type that drives scarring of the kidney – the myofibroblast – and the cell that causes its activation – proximal tubule – will reveal pathways for drug intervention to prolong kidney function. The pathways for which drugs already exist for other indications will then be tested to slow progression of the disease in a mouse model of Alport syndrome.
The following research projects were funded by Alport Syndrome Foundation, the Pedersen Family, and the Kidney Foundation of Canada (KFOC):
AWARDED 2018
Investigation of Cochlear Pathology and Hearing Loss in a Mouse Model of Alport Syndrome
Dr. Peter Santi & Dr. Cliff Kashtan (University of Minnesota)
The ways in which AS affects hearing remains a mystery, one researchers from the University of Minnesota are hoping to better understand through a groundbreaking, investigational study. For their research, Drs. Peter Santi and Cliff Kashtan will be examining the hearing and cochlear microstructures of AS mice at various stages of development. The results of the study could reveal new potential alternatives for alleviating hearing loss in AS patients, other than the use of hearing aids.
Modeling Alport Syndrome (AS) using iPSC-derived glomeruli
Dr. Melissa Little (Royal Children’s Hospital) & Dr. Rachel Lennon (The University of Manchester)
This ASF-funded study takes a closer look at the cells of the kidney. Drs. Melissa Little from Royal Children’s Hospital and Rachel Lennon from the University of Manchester have developed a new way to grow functioning “filter cells” of the kidney in a petri dish, starting with the cells of any healthy human being. In this study, the researchers will try to grow AS-afflicted cells, using cells from a patient with AS. The study may help researchers understand how collagen IV is misassembled in the kidneys of AS patients, without the need for patients to undergo biopsies. If successful, results of the experiment may result in a faster method of testing potential new drug therapies for AS patients.
AAV2-CRiSPR/Cas9 preclinical trial on a naturally occurring ATS dog model
Dr. Alessandra Renieri (University of Siena) & Dr. Mary Nabity (Texas A&M University)
This study, led by Dr. Alessandra Renieri from the University of Siena and Dr. Mary Nabity from Texas A&M University, is the first proof-of-concept study to test gene therapy as a treatment for AS. For this study, the doctors will use a powerful new DNA editing tool to try to correct the specific Col4a5 mutation of a patient with AS. They’ll do this by editing the DNA of a single kidney cell collected from the patient’s urine— leaving the rest of the DNA untouched. The corrected genes would then be put into something similar to a live vaccine. The patient’s kidney would then be “infected” with this vaccine. If the corrected genes survive, they may, in turn, cure the patient of AS.
AWARDED 2017
Targeting podocyte lipotoxicity in Alport syndrome
Dr. Alessia Fornoni, University of Miami (Florida)
Dr. Alessia Fornoni was awarded $100,000 for a 15-month study on Targeting podocyte lipotoxicity in Alport syndrome. In Alport syndrome, mutations in type IV collagen genes lead to abnormal collagens being expressed in kidney basement membranes. These abnormal collagens activate receptors called DDR1 and CD36 that lead to uptake of fatty acids and cholesterol into podocytes, an important cell attached to the basement membrane, and injury to this cell. This research project aims to understand this injury pathway further through studies in cell culture and also aims to prevent the progression of kidney disease in mice with Alport syndrome by blocking the uptake of fatty acids and cholesterol into podocytes by treatment with a currently FDA-approved drug, Ezetimibe.
Click here to read Dr. Fornoni’s full research abstract.
Repurposing of FDA approved chemical chaperones to the rescue of a mouse model of Alport syndrome
Dr. Constantinos Deltas, University of Cyprus (Cyprus)
Dr. Constantinos Deltas was awarded $100,000 for an 18-month study on Repurposing of FDA approved chemical chaperones to the rescue of a mouse model of Alport syndrome. In some patients with Alport syndrome a missense mutation leads to abnormal folding of the type IV collagen protein and breakdown of the protein before it gets to the basement membrane. This research project aims to use chaperone medicines called PBA and TUDCA to help the abnormally folded type IV collagen make it to the basement membrane intact in a mouse model of Alport syndrome. Having any type IV collagen in the basement membrane, even it if has a minor missense mutation, is hypothesized to improve the long-term kidney outcome of the disease.
Click here to read Dr. Deltas’s full research abstract.
AWARDED 2016
Search for therapeutic reagents by modeling Alport syndrome in mice and humans
Dr. Hirofumi Kai, Kumamoto University (Japan)
Dr. Hirofumi Kai was awarded $100,000 for a two-year study on the Search for therapeutic reagents by modeling Alport syndrome in mice and humans. The study aims to accelerate the understanding of the progression of Alport syndrome by investigating chemical compounds that induce COL4A3/4/5 formation; identifying key factors in podocytes upstream (modulators) and downstream (effectors) of p53 (tumor suppressor gene that has a protective effect on the kidney in Alport syndrome mice models); and validating findings using induced pluripotent stems cells (iPS cells) from people who have Alport syndrome.
Click here to read Dr. Kai’s full research abstract.
5-Ht2b Antagonism As A Strategy To Prevent Renal Function Loss In Alport syndrome
Dr. Jeffrey Miner, Washington University (Missouri, USA)
Dr. Jeffrey Miner was awarded $100,000 for a one-year study to investigate 5-Ht2b Antagonism As A Strategy To Prevent Renal Function Loss In Alport syndrome. This research study seeks to prove that preventing activation of the serotonin receptor 2B (also known as 5-HT2B) will reduce kidney fibrosis (scarring), slow kidney disease progression, and cooperate with ACE inhibition to prolong kidney function in Alport mice. This approach will be to “repurpose” a drug that has already been shown to be safe in humans in Phase I clinical trials.
Click here to read Dr. Miner’s full research abstract.
AWARDED 2015
Drug Repurposing for the Treatment of Experimental Alport syndrome
Dr. James Scholey, University of Toronto (Ontario, Canada)
Dr. James Scholey was awarded $100,000 for a one-year study on Drug Repurposing for the Treatment of Experimental Alport syndrome. The study will target the patterns of gene expression in the kidney associated with the progression of kidney injury using the FDA-approved drug vorinostat . It will be determined if this drug prolongs the lifespan of mice and if combined treatment with ACE inhibitors provides additional benefit.
Click here to read Dr. Scholey’s full research abstract.
WISE Antibody as a Treatment for Alport syndrome
Dr. Jeffrey Miner, Washington University (Missouri, USA)
Dr. Jeffrey Miner was awarded $76,500 for a one-year study on WISE Antibody as a Treatment for Alport syndrome. The goal of this study is to test the effectiveness of 2 antibodies at slowing the progression of kidney failure both separately and with ACE inhibition in mice models.
Click here to read Dr. Miner’s full research abstract.
AWARDED 2014
Correction of the genetic defect in Alport syndrome using the TALEN approach
Dr. Judy Savige, University of Melbourne (Australia)
Dr. Sharon Ricardo, Monash University (Australia)
Dr. Judy Savige and Dr. Sharon Ricardo were awarded $100,000 for a one-year study on Correction of the genetic defect in Alport syndrome using the TALEN approach. This research study will attempt to repair the genetic mutations in cell lines from patients with Alport syndrome due to missense and nonsense mutations, confirm that these mutations are corrected in vitro and that the mutation is repaired, as well as to determine any increase in cell stress or apoptosis.
Click here to read Dr. Savige‘s and Dr. Ricardo‘s full research abstract.
Podocyte response to injury in Alport syndrome: an answer from human amniotic fluid kidney progenitors
Dr. Stefano Da Sacco, Saban Research Institute at Children‘s Hospital Los Angeles (California, USA)
Dr. Da Sacco was awarded $100,000 for a one-year study on Podocyte response to injury in Alport syndrome: an answer from human amniotic fluid kidney progenitors. The study will evaluate recently identified renal progenitors from human amniotic fluid that can be differentiated in vitro into mature and functional podocytes. The physiology and pathology of the podocytes will be investigated in order to better understand the response of these cells to therapeutic compounds.
Click here to read Dr. Da Sacco‘s full research abstract.
AWARDED 2013
Nephroprotective and antifibrotic potential of microRNA-21 in the COL4A3 knockout mouse model of Alport syndrome
Dr. Oliver Gross, University Medical Centre Göttingen (Germany)
Dr. Oliver Gross was awarded $98,500 for a one year study on the Nephroprotective and antifibrotic potential of microRNA-21 in the COL4A3 knockout mouse model of Alport syndrome. The research project goals are to evaluate anti microRNA-21 therapy in reducing renal fibrosis and promoting podocyte functioning.
Click here to read Dr. Gross‘s full research abstract
Derivation and Characterisation of induced pluripotent stem cell lines from patients with X-Linked Alport syndrome — a model for examining mechanisms and therapies
Dr. Judy Savige, University of Melbourne (Australia)
Dr. Sharon Ricardo, Monash University (Australia)
Dr. Judy Savige and Dr. Sharon Ricardo were awarded $98,600 for a one year study on the Derivation and Characterisation of induced pluripotent stem cell lines from patients with X-Linked Alport syndrome ““ a model for examining mechanisms and therapies. The research project will use reprogrammed adult stem cells to generate podocytes which will be examined to evaluate how Alport syndrome mutations are produced and to investigate treatments targeting these mutations.
Click here to read Dr. Savige‘s and Dr. Ricardo‘s full research abstract
AWARDED 2012
Defining Efficacy of Combination Drug Therapy in Alport Mice
Dr. Jeffrey Miner, Washington University (Missouri, USA)
Dr. Miner was awarded $100,000 for a one year study on Defining Efficacy of Combination Drug Therapy in Alport Mice. The goal of this proposal is to determine whether combined therapy with both and ACE inhibitor and a proprietary inhibitor of chemokine receptor 2 and 5 (CCR2 and CCR5) activation will have a synergistic effect at slowing Alport disease progression and will extend life span even more than the ACE inhibitor alone.
Click here to read Dr. Miner‘s full research abstract
Click here to read about Dr. Miner‘s recent research on gene therapy
AWARDED 2011
Amniotic Fluid Stem Cells (AFSC) and Alport syndrome
Dr. Laura Perin, Saban Research Institute at Children‘s Hospital Los Angeles (California, USA)
Dr. Perin was awarded $100,000 for a two-year research project on Amniotic Fluid Stem Cells (AFSC) and Alport syndrome. Dr. Perin proposes to use Amniotic Fluid Stem Cells (AFSC) as a novel approach to reversing fibrosis in Alport syndrome.
Click here for more information on Amniotic Fluid Stem Cells (AFSC)
Click here for to read Dr. Perin‘s full research abstract
Patients play a key part in supporting research by:
- Raising funds as campaigners for the Annual Campaign
- Registering with the patient registry to expand data available to researchers regarding the number of patients diagnosed, disease progression, genetics types, and other valuable patient histories that remain private and confidential through the registry. Patients in the registry are also kept informed of eligible upcoming studies and clinical trials. Interested? Contact ASF Staff.
- Participating in studies and clinical trials.