
Goal Exceeded – Thank YOU!
 We appreciate our community supporting our largest Annual Campaign goal to date.
We appreciate our community supporting our largest Annual Campaign goal to date.
ASF’s 4-week 2022 Annual Campaign raised a total of $187,684, exceeding the goal by more than $10,000.
We are grateful to the nearly 400 generous donors, as well as the 28 volunteer campaigners, whose leadership played a major role in this success.
We extend our gratitude to all those who participated with gifts of all sizes and/or watched our educational videos that led to dollars per view by an anonymous donor.
We remain focused and dedicated to meeting the needs of patients and families, advancing research that can improve lives, and helping build a pipeline toward potential treatments and a cure.
ASF’s 2022 Annual Report, including Annual Campaign details, will be available in early 2023.
Mental Health Spotlight, New Coping Resources
 With guidance and input from several patient volunteers who are also mental health professionals, ASF has created a new webpage containing mental health resources for Alport patients and families.
With guidance and input from several patient volunteers who are also mental health professionals, ASF has created a new webpage containing mental health resources for Alport patients and families.
During last month’s virtual Direct Connect meeting, participants shared experiences related to mental health and living with Alport syndrome. Examples include:
Emotions stemming from initial misdiagnosis (anger, anxiety, confusion, grief)
Guilt over passing on a genetic condition to children
Not wanting to overburden others by sharing ongoing concerns
Symptoms and/or questions being “brushed off” by health care providers and resulting in patient frustration
Similar experiences are frequently expressed by individuals that reach out to ASF, complete our patient surveys, and arise in conversations at patient meetings. Feelings of anxiety related to living with Alport syndrome are also documented in the 2019 Voice of the Patient Report, which summarizes findings from the Externally-Led Patient Focused Drug Development meeting held to inform the FDA about the unmet needs of the Alport syndrome community.
New AFFINITY Study Educational Video
The AFFINITY study, sponsored by Chinook Therapeutics, is a phase 2 clinical trial studying the safety and efficacy of atrasentan in proteinuric glomerular diseases, including Alport syndrome.
In this new closed captioned video, Dr. Sreedhar Mandayam, Professor of Medicine and Nephrologist at the University of Texas MD Anderson Cancer Center, discusses the AFFINITY study’s trial design and eligibility criteria, what participants can expect during the trial, and more.
To learn more about the AFFINITY study, including active study sites, visit our website.
Education & Awareness for Doctors, Researchers
 Earlier this month, ASF was well-represented at the American Society of Nephrology’s Kidney Week 2022.
Earlier this month, ASF was well-represented at the American Society of Nephrology’s Kidney Week 2022.
Approximately 10,000 nephrologists were in attendance. Our exhibit booth provided educational materials for medical professionals and researchers. Members of our Medical Advisory Committee and Scientific Advisory Research Network in attendance were able to connect with one another in person. We met with pharmaceutical and biotech partners exploring Alport syndrome and/or offering beneficial services for patients.
A highlight was ASF’s Board Member and Chair of our Research Program, Dr. André Weinstock (pictured top, center), giving a featured presentation about the importance of genetic testing in Alport syndrome from the perspective of patients and ASF.
Overall, excellent connections were made for future directions of research!
Clinical Trial Adds Two Study Sites
R3R01-ASFSGS-201 is a phase 2 clinical trial testing an investigational medication called R3R01. R3R01 has the potential to benefit patients with Alport syndrome and other glomerular diseases by preserving or improving kidney function and reducing the amount of protein in the urine.
Study sponsor, River 3 Renal Corp., recently announced the addition of two new sites. The Principal Investigators at these sites are Alport experts that serve on ASF’s Medical Advisory Committee, Dr. Michelle Rheault at University of Minnesota and Dr. James Simon at Cleveland Clinic.
|
|
Additional sites can be found in California, Florida, Ohio, and Texas. To learn more about the study, including all site locations, visit our Clinical Trials page.

ASF Website: Updates to Improve User Experience
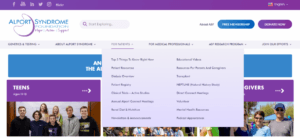 In the interest of making the ongoing expansion of website content more readily-accessible, the alportsyndrome.org home page has been altered to include large dropdown menus for topic-based categories when viewed on a desktop or laptop.
In the interest of making the ongoing expansion of website content more readily-accessible, the alportsyndrome.org home page has been altered to include large dropdown menus for topic-based categories when viewed on a desktop or laptop.
Mobile users can access the topic categories by clicking the 3 stacked vertical lines at the top right of the page. Our social media hyperlinks and language translation tool have also been relocated for quicker browser access.
View our Newsletters and Announcements page for an archive of prior communications.

